Sulphide ore of copper are (a) Copper glance `(Cu_(2)S)` and (b) Copper pyrites `(CuFeS_(2))`. Copper is obtained in pure form from its sulphide ore in the following steps:
1. Concentration of ore through froth floatation method : In this method gangue is removed from the sulphide ore. The finely crushed ore is concentrated by froth-floatation process. The finely crushed ore is suspended in water containing a little amount of pine oil. A blast of air is passed through the suspension. The particles get wetted by the oil and float as a froth which is skimmed. The gangue (unwanted materials) sinks to the bottom, and is then removed.
2. Conversion of sulphide ore to oxide by roasting : The concentrated ore is then roasted in a furnace between `500^(@)C` and `700^(@)C` in the presence of a current of air, Sulphur is oxidised to `SO_(2)` and other impurities are also removed as volatile oxides. The following reaction takes place.
`2CuFeS_(2) + O_(2) to Cu_(2)S +2FeS +SO_(2)`
`2Cu_(2)S +3O_(2) to 2Cu_(2)O +2SO_(2)`
`2FeS +3O_(2) to 2FeO +2SO_(2)`
`FeO + SiO_(2) to FeOSiO_(3)`
3. Extraction of copper from cuprous oxide: Auto reduction of cuprous oxide takes place in Bessemer converter.
`2Cu_(2)O +underset("Blister copper")(Cu_(2)S to 6Cu) +SO_(2)uarr`
4. Refining: Finally pure copper is obtained through the electrolysis of the above obtained copper.
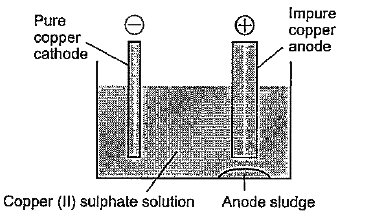
Blister copper is refined by electrolysis. Blocks of blister copper are casted to use as anode and thin sheets of pure copper act as cathode. The cathode plates are coated with graphite in order to remove depositing copper. The electrolyte is copper sulphate `(CuSO_(4))` mixed with a little amount of `H_(2)SO_(4)` to increase the electrical conductivity. Optimum potential difference is 1.3 volt for this electrolytic process. During electrolysis, pure copper (99.99% Cu) is deposited on the cathode plates and impurities which are soluble fall to the bottom of the cell as anode mud or sludge.
`Cu to Cu^(2+) +2e^(-)" "` (at the anode)
`Cu^(2+) +2e^(-) to Cu" "` (at the cathode)
This electrically refined copper is 100% pure.