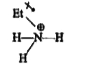Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
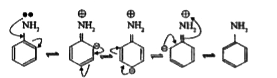
- State reasons for the following: Aliphatic amines are stronger bas...
Text Solution
|
- निम्नलिखित में प्रत्येक का संभावित कारण बताइए- (i)समतुल्य अणु द...
Text Solution
|
- निम्नलिखित में प्रतयेक का संभावित कारण बताइएः (i) समतुल्य अणु द्रव्य...
Text Solution
|
- निम्नलिखित में प्रत्येक का संभावित कारण बताइए - (i) समतुल्य अण...
Text Solution
|
- संभावित कारण बताय --- एरोमेटिक एरोमेटिक की तुलना में एलफितिक एमिनों प...
Text Solution
|
- Aliphatic amines are more basic than aromatic amines. Explain
Text Solution
|
- Give plausible explanation for each of the following : Why are aliph...
Text Solution
|
- Aliphatic amines are stronger bases than ammonia due to of alkyl grou...
Text Solution
|
- Why are primary aliphatic amines stronger bases than ammonia?
Text Solution
|