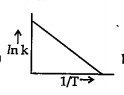
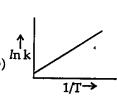
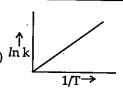
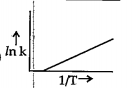
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- According to Arrhenius equation rate constant k is equal to Ae^(-Ea//R...
Text Solution
|
- According to Arrhenius equation rate constant k is equal to Ae^(-E(a)/...
Text Solution
|
- According to Arrheneius equation rate constant k is equal to Ae^(-E(a)...
Text Solution
|
- According to Arrhenius equation rate constant K is equal to A e.^(-...
Text Solution
|
- According to Arrhenius equation, rate constant k is equal to "A e"^(-E...
Text Solution
|
- According to Arrhenius equations rate constant (k) is equal to Ae^(-E(...
Text Solution
|
- According to Arrhenius equation rate constant K is equal to A e.^(-...
Text Solution
|
- According to Arrhenius equation rate constant k is equal to A e^(-E(a)...
Text Solution
|
- According to Arrhenius equation rate constant k is equal to Ae^(-E(a)/...
Text Solution
|