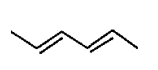
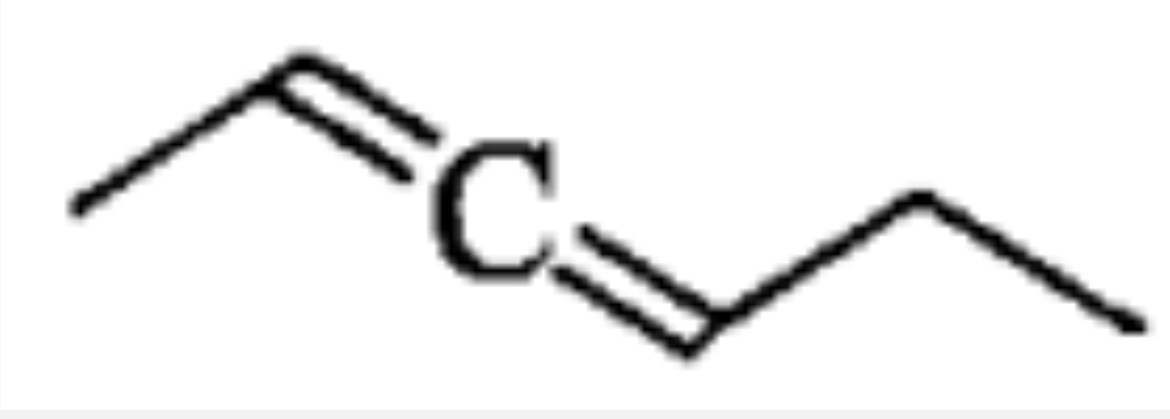
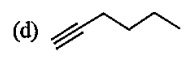
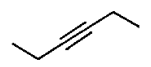A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An organic compound X with molecular formula C6H10, when treated with ...
Text Solution
|
- An organic compound 'X' having molecular formula C(5)H(10)O yield phen...
Text Solution
|
- An organic compound 'X' having molecular formula C(5)H(10)O yield phen...
Text Solution
|
- An organic compound 'X' having molecular formula C(5)H(10)O yield phen...
Text Solution
|
- The compounds which react with dilute H2SO4 in presence of HgSO4 to ...
Text Solution
|
- When acetylene is passed through dil.H2SO4 in presence of HgSO4,the co...
Text Solution
|
- A neutral compound with molecular formula C(3)H(8)O evolves H(2) when ...
Text Solution
|
- An organic compound 'X' having molecular formula C(5)H(10) yields phen...
Text Solution
|
- When acetylene is passed through dil .H2SO4 in the presence of HgSo4 t...
Text Solution
|