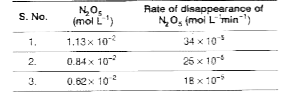Topper's Solved these Questions
CHEMICAL KINETICS
ARIHANT PUBLICATION|Exercise QUESTIOS FOR PRACTICE (Part III Integrated Rate Equations) Multiple Choice Type Questions|4 VideosCHEMICAL KINETICS
ARIHANT PUBLICATION|Exercise QUESTIOS FOR PRACTICE (Part III Integrated Rate Equations) Very Short Answer Type Questions|5 VideosCHEMICAL KINETICS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR ASSESSMENT (Part II Molecularity and Order of a Chemical Reaction) Short Answer Type I Questions|3 VideosCARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |3 VideosCHEMISTRY IN EVERYDAY LIFE
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |2 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-CHEMICAL KINETICS-QUESTIONS FOR ASSESSMENT (Part II Molecularity and Order of a Chemical Reaction) Short Answer Type II Questions
- Distinguish between order and molecularity.
Text Solution
|
- Why is the probability of reaction with molecularity higher than three...
Text Solution
|
- The data given below is for the reaction, 2N(2) O(g) (g) to 4NO(2) (...
Text Solution
|
- The data given below is for the reaction, 2N(2) O(g) (g) to 4NO(2) (...
Text Solution
|
- The data given below is for the reaction, 2N(2) O(g) (g) to 4NO(2) (...
Text Solution
|