Topper's Solved these Questions
CHEMICAL KINETICS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Short Answer Type II Questions)|8 VideosCARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |3 VideosCHEMISTRY IN EVERYDAY LIFE
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |2 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-CHEMICAL KINETICS-CHAPTER PRACTICE (Long Answer Type Questions)
- Hydrogen peroxide, H(2) O(2) (aq) decomposes to H(2) O(l) and O(2) (g)...
Text Solution
|
- Hydrogen peroxide, H(2) O(2) (aq) decomposes to H(2) O(l) and O(2) (g)...
Text Solution
|
- Answer the following questions on the basis of the curve given below f...
Text Solution
|
- Answer the following questions on the basis of the curve given below f...
Text Solution
|
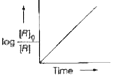
- Consider the reaction, A overset(k) (to) B. The change in concentratio...
Text Solution
|
- Consider the reaction, A overset(k) (to) B. The change in concentratio...
Text Solution
|
- Consider the reaction, A overset(k) (to) B. The change in concentratio...
Text Solution
|
- What are the conditions for a collision to be effective.
Text Solution
|