Text Solution
Verified by Experts
Topper's Solved these Questions
ELEMENTS : NITROGEN FAMILY
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE ( Short Answer Type II Questions ) |17 VideosELEMENTS : NITROGEN FAMILY
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE ( Long Answer Type Questions ) |1 VideosELEMENTS : NITROGEN FAMILY
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE ( Very Short Answer Type Questions ) |19 VideosELECTROCHEMISTRY
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE ( LONG ANSWER TYPE QUESTIONS) |3 VideosETHERS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (7 MARK)|3 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-ELEMENTS : NITROGEN FAMILY-QUESTIONS FOR PRACTICE ( Short Answer Type Questions )
- Explain why NH(3), is basic, while BiH(3) , is only feebly basic?
Text Solution
|
- Account for the Bi(V) is a stronger oxidising agent than Sb(V).
Text Solution
|
- Account for the N-N single bond is weaker than P- P bond.
Text Solution
|
- Why does NH3 form hydrogen bond but PH3 does not ?
Text Solution
|
- Why PH(3), has lower boiling point than NH(3)?
Text Solution
|
- Why is PCI5, more covalent than PCI3 ?
Text Solution
|
- Explain, why all the bonds In PCI5are not identical.
Text Solution
|
- Why does nitrogen show catenation property less than phosphorus?
Text Solution
|
- Unlike phosphorus, nitrogen shows little tendency for catenation. Why?
Text Solution
|
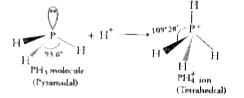
- Bond angle in PH(4)^(+) is higher than that in PH(3). Why?
Text Solution
|
- lllustrate how copper metal can give different products on reaction wi...
Text Solution
|
- Nitric oxide becomes brown when released in air. Why?
Text Solution
|
- BiCl(3), is more stable than BiCl(5) Why?
Text Solution
|
- In the ring test of NO(3)^(-) ion, nitrate ion reduces to nitric oxide...
Text Solution
|
- On reaction with Cl(2) , phosphorus forms two types of halides 'A' and...
Text Solution
|
- What happens when PCl(5) is heated ?
Text Solution
|
- What happens when H(3)PO(3) is heated ?
Text Solution
|