Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part I Short Answer Type II Questions 3 Marks|22 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part I Long Answer Type Questions 7 Marks|6 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part I Very Short Answer Type Questions 1 Mark |17 VideosCHEMISTRY IN EVERYDAY LIFE
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |2 VideosD-BLOCK ELEMENTS
ARIHANT PUBLICATION|Exercise Chapter practice (Long answer type questions)|2 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-COORDINATION COMPOUNDS -QUESTIONS FOR PRACTICE Part I Short Answer Type I Questions 2 Marks
- What is meant by the chelate effect? Give an example.
Text Solution
|
- Amongst the following, the most stable complex is (i) Fe(H(2)O)(6)]^...
Text Solution
|
- Write t he IUPAC name of the following complex. [Cr(NH(3))(2)Cl(2)("...
Text Solution
|
- Write the formula for the following complex. Pentaamminenitrito-O--c...
Text Solution
|
- Name the type of isomerism when ambidentate ligands are attached to ce...
Text Solution
|
- Write the IUPAC name of the complex [Cr(NH(3))(4)Cl(2)]^(+). What type...
Text Solution
|
- Give the evidence that [Co(NH(3))(5)Cl]SO(4)and[Co(NH(3))(5)SO(4)]Cl a...
Text Solution
|
- A complex of the type [M(A A)(2)X(2)]^(n+) is known to be optically ac...
Text Solution
|
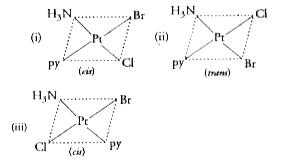
- Write the geometrical isomers of [Pt(NH(3))(Br)(Cl)("py")] and how man...
Text Solution
|
- FeSO4, solution mixed with (NH4)2SO4, solution in 1:1 molar ratio give...
Text Solution
|
- A coordiantion compound CrCl(3)*4H(2)O percipitates silver chloride wh...
Text Solution
|