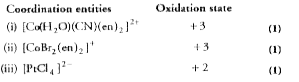Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part I Long Answer Type Questions 7 Marks|6 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR ASSESSMENT Part I Multiple Choice Type Questions 1 Mark |2 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part I Short Answer Type I Questions 2 Marks|11 VideosCHEMISTRY IN EVERYDAY LIFE
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |2 VideosD-BLOCK ELEMENTS
ARIHANT PUBLICATION|Exercise Chapter practice (Long answer type questions)|2 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-COORDINATION COMPOUNDS -QUESTIONS FOR PRACTICE Part I Short Answer Type II Questions 3 Marks
- Explain with two examples each of the following. Coordiantion entity, ...
Text Solution
|
- What is meant by unidentate, didentate and ambidentate ligand? Give tw...
Text Solution
|
- Specify the oxidation number of metal in the following coordination en...
Text Solution
|
- Specify the oxidation number of metal in the following coordination en...
Text Solution
|
- Specify the oxidation number of metal in the following coordination en...
Text Solution
|
- Explain the bonding in coordination compounds in terms of Werner's pos...
Text Solution
|
- Using IUPAC norms, write the systematic names of the following. [Co(...
Text Solution
|
- Using IUPAC norms, write the systematic names of the following. [Pt(...
Text Solution
|
- Using IUPAC norms, write the systematic names of the following. [Ti(...
Text Solution
|
- Write the formulae for the following coordination compounds. Tetraam...
Text Solution
|
- Write the formulae for the following coordination compounds. Potassi...
Text Solution
|
- Write the formulae for the following coordination compounds. Ammineb...
Text Solution
|
- Write the formulae for the following coordination compounds. Dichlor...
Text Solution
|
- Write the formulae for the following coordination compounds. Iron(II...
Text Solution
|
- Write the formulae for the following coordination compounds.
Text Solution
|
- Write the types of isomerism exhibited by the following complexes. [...
Text Solution
|
- Write the types of isomerism exhibited by the following complexes. [...
Text Solution
|
- Write the types of isomerism exhibited by the following complexes. [...
Text Solution
|
- What type of isomerism is exhibited by [Co(NH(3))(4)Cl(2)]^(+)Br^(-)? ...
Text Solution
|
- Draw all the isomers (geometrical and optical) of [CoCl(2)("en")(2)]...
Text Solution
|