Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part II Long Answer Type Questions 7 Marks |9 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR ASSESSMENT Part II Multiple Choice Type Questions 1 Mark |2 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part II Short Answer Type I Questions 2 Marks |9 VideosCHEMISTRY IN EVERYDAY LIFE
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |2 VideosD-BLOCK ELEMENTS
ARIHANT PUBLICATION|Exercise Chapter practice (Long answer type questions)|2 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-COORDINATION COMPOUNDS -QUESTIONS FOR PRACTICE Part II Short Answer Type II Questions 3 Marks
- Expalin the basis of valence bond theory that [Ni(CN)(4)]^(2-) ion wit...
Text Solution
|
- Explain [Co(NH(3))(6)]^(3+) is an inner orbital complex whereas [Ni(NH...
Text Solution
|
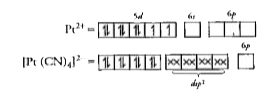
- Predict the number of unpaired electrons in the square planar [Pt(CN)(...
Text Solution
|
- Write the assumptions of crystal field theory. Discuss the patterm of ...
Text Solution
|
- What is spectrochemical series? Explain the difference between a weak ...
Text Solution
|
- What is the crystal field stabilisation energy? How does the magnitude...
Text Solution
|
- What is meant by crystal field splitting energy? On the basis of cryst...
Text Solution
|
- The haexaaquamanganese (II) ion contains five unpaired electrons, whil...
Text Solution
|
- Give the electronic configuration of the following complexes on the ba...
Text Solution
|
- Amongst the following ions which one has the highest magnetic moment v...
Text Solution
|
- [Fe(CN)6]^(4-) and [Fe(H2O)6]^(2+), are of different colours in dilate...
Text Solution
|
- A solution [Ni(H2O)6]^(2+) is green while a solution of [Ni(CN)4]^(2-)...
Text Solution
|