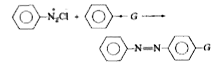
Text Solution
Verified by Experts
Topper's Solved these Questions
AMINES
ARIHANT PUBLICATION|Exercise Odisha Bureau.s Textbook Solutions (Short Answer Type II Questions)|23 VideosAMINES
ARIHANT PUBLICATION|Exercise Odisha Bureau.s Textbook Solutions (Long Answer Type Questions)|24 VideosAMINES
ARIHANT PUBLICATION|Exercise Odisha Bureau.s Textbook Solutions (Fill in the Blanks)|5 VideosALCOHOLS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Long Answer Type Questions)|4 VideosARYL DIAZONIUM SALTS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) 7 MARKS |2 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-AMINES-Odisha Bureau.s Textbook Solutions (Short Answer Type I Questions)
- How will you obtain methanol from methyl amine?
Text Solution
|
- How can you get methylamine from ethylamine?
Text Solution
|
- Give the IUPAC name of the following compounds CH(3)CH(2)CH(2)NH(2)
Text Solution
|
- Give the IUPAC name of the following compounds CH(3)NHCH(2)CH(3)
Text Solution
|
- Give the IUPAC name of the following compounds (CH(3))(2)CHNH(2)
Text Solution
|
- Give the IUPAC name of the following compounds (CH(3))(3)C.NH(2)
Text Solution
|
- Give the IUPAC name of the following compounds (CH(3))(2)NCH(2)CH(2)...
Text Solution
|
- Give the IUPAC name of the following compounds CH(3)CH(2)CH(NH(2))CH...
Text Solution
|
- Give the structural isomers of C(3)H(9)N and C(4)H(11)N and give their...
Text Solution
|
- Name the functional group in the following compounds. CH(3)CH(2)NH(2) ...
Text Solution
|
- What happens when nitromethane is reduced ? Give equation.
Text Solution
|
- Name the reagent used to distinguish between ethyl amine and diethyl a...
Text Solution
|
- A compound with molecular formula CH(5)N on treatment with HNO(2) libe...
Text Solution
|
- How you will distinguish C(2)H(5)NH(2) and C(6)H(5)NH(2)?
Text Solution
|
- What is carbylamine test?
Text Solution
|
- Why is aniline less basic than ammonia ?
Text Solution
|
- Why is ethyl amine more basic than ammonia ?
Text Solution
|
- How will you carry out the conversion of benzene to p-nitroaniline?
Text Solution
|
- Direct nitration of aniline is not carried out at all. Explain Why?
Text Solution
|
- Give an example of a Zwitter ion.
Text Solution
|