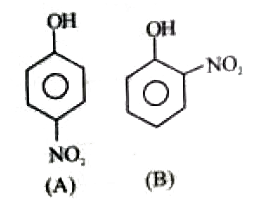
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-SOLUTIONS-NEET PAST 5 YEARS QUESTIONS
- Out of the compounds given below , the vapour presssure of (B) at a pa...
Text Solution
|
- The mixture which shows positive deviation from Raoult's law is:
Text Solution
|
- The freezing point depression constant of benzene is 5.12 K kg mol^(-1...
Text Solution
|
- If 8g of a non-electrolyte solute is dissolved in 114g of n-octane to ...
Text Solution
|
- Isotonic solutions have same
Text Solution
|
- For an ideal solution, the correct option is:
Text Solution
|
- The mixture that forms maximum boiling azeotrope is :
Text Solution
|
- If molarity of the dilute solutions is doubled ,the value of molal dep...
Text Solution
|