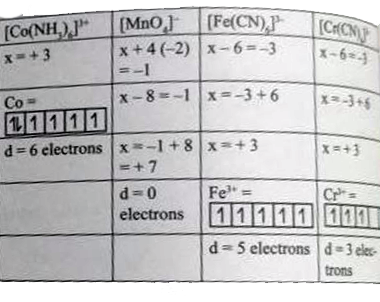
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
PHYSICS WALLAH|Exercise LEVEL-2|27 VideosCOORDINATION COMPOUNDS
PHYSICS WALLAH|Exercise NEET PAST 5 YEARS QUESTIONS |8 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PHYSICS WALLAH|Exercise NEET PAST 5 YEARS QUESTIONS |2 VideosELECTROCHEMISTRY
PHYSICS WALLAH|Exercise NEET PAST 5 YEARS QUESTIONS |32 Videos
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-COORDINATION COMPOUNDS -NEET PAST 5 YEARS QUESTIONS
- The complex ion which has no 'd'-electrons in the central metal atom i...
Text Solution
|
- Pick out the correct statement with respect to [Mn(CN)(6)]^(3-):
Text Solution
|
- The correct order of the stoichiometries of AgCl formed when AgNO3 in ...
Text Solution
|
- Which of the following complex ions is not diamagnetic?
Text Solution
|
- The electron distribution in d' coordination complexes depends on magn...
Text Solution
|
- The [Co(H(2)O)(6)]^(2+) ion has three unpaired electrons. The hybridis...
Text Solution
|
- The correct increasing order of trans-effect of the following species ...
Text Solution
|
- Jahn - Teller effect is not observed in high spin complexes of
Text Solution
|
- Which of the following has longest C-O bond length? (Free C-O bond len...
Text Solution
|