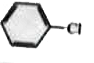
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-HALOALKANES AND HALOARENES -NEET PAST 5 YEARS QUESTIONS
- Elimination reaction of 2-Bromo -pentane to form pent-2-ene is (1)b...
Text Solution
|
- Which of the following will not undergo SN^1 reaction with OH^-
Text Solution
|
- An example of a sigma bonded organometallic compound is:
Text Solution
|
- Which of the following will react faster through SN 1 mechanism ?
Text Solution
|
- From amongest the following alcohols, the one that would react fastest...
Text Solution
|
- Consider the reaction : CH(3)CH(2)CH(2)Br+NaCNrarrCH(3)CH(2)CH(2)CN+...
Text Solution
|
- Which of the following biphenyls is optically active?
Text Solution
|