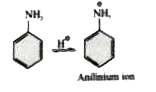
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-AMINES -NEET Past 5 Years Questions
- Which of the following amine will give the carbylamine test ?
Text Solution
|
- Reaction of propanamide with ethanolic sodium hydroxide and bromine wi...
Text Solution
|
- The correct order of the basic strength of methyl substituted amines i...
Text Solution
|
- Nitration of aniline in strong acidic medium also gives m-nitroaniline...
Text Solution
|
- The correct increasing order of basic strength for the following compo...
Text Solution
|
- Which of the following reactions is appropriate for converting acetami...
Text Solution
|
- The reaction: ArN2Cl overset(Cu//HCl)rarrArCl +N2 + CuCl is known as:...
Text Solution
|
- The product (P) of the following reaction
Text Solution
|
- A given nitrogen-containing aromatic compound A reacts with Sn/HCI, fo...
Text Solution
|
- Which one of the following -compounds does not react with nitrous acid...
Text Solution
|
- The correct statement regarding the basicity of arylamines is .
Text Solution
|