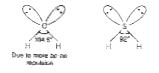Text Solution
Verified by Experts
Topper's Solved these Questions
GROUP 16 ELEMENTS: OXYGEN FAMILY
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (LONG ANSWER TYPE QUESTIONS)|11 VideosGROUP 16 ELEMENTS: OXYGEN FAMILY
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS (A MULTIPLE CHOICE TYPE QUESTIONS)|30 VideosGROUP 16 ELEMENTS: OXYGEN FAMILY
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (VERY SHORT ANSWER TYPE QUESTIONS)|26 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Long Answer Type Questions )|4 VideosGROUP 17 ELEMENTS : HALOGEN FAMILY
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Long Answer Type Questions)|1 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-GROUP 16 ELEMENTS: OXYGEN FAMILY-QUESTIONS FOR PRACTICE (SHORT ANSWER TYPE I QUESTIONS)
- SF6 is known but SCl6 is not. Why?
Text Solution
|
- SF6 is inert towards hydrolysis. Why?
Text Solution
|
- Out of H2O and H2S, which one has higher bond angle and why?
Text Solution
|
- What is the action of ozone with lead sulphide? Give equation.
Text Solution
|
- How does Ozone react with KI solution ?
Text Solution
|
- What happens when O3 is passed through acidified SnCl2 solution ?
Text Solution
|
- Why is ozone not collected over mercury?
Text Solution
|
- Complete the equation given below and make it a balanced equation Sn...
Text Solution
|
- What happens when SO2gas is passed through lime water first slowly and...
Text Solution
|
- What happens when SO2 is passed through sulphuretted-hydrogen dissolve...
Text Solution
|
- Why hydrogen sulphide cannot be dried by conc. H2SO4?
Text Solution
|
- How do the following pairs of compounds react? H2S+SO2 rarr
Text Solution
|
- What happens when Sulphur dioxide is passed through FeCl3 solution ?
Text Solution
|
- Explain bleaching action of sulphur dioxide.
Text Solution
|
- How is the presence of SO2 detected?
Text Solution
|
- Comment on the nature of two S-O bonds formed in SO2 molecule. Are the...
Text Solution
|
- What happens when potassium bromide is treated with warm conc. sulphur...
Text Solution
|
- What happens when copper is heated with concentrated H2SO4? Give equat...
Text Solution
|
- Why is K(a2) lt lt K(a1) for H2SO4 in water?
Text Solution
|
- Justify the placement of O, S, Se, Te and Po in the same group of the ...
Text Solution
|