Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMISTRY IN EVERYDAY LIFE
ARIHANT PUBLICATION|Exercise CHEMICAL IN INDUSTRIES (QUESTIONS FOR PRACTICE (LONG ANSWER TYPE QUESTIONS))|3 VideosCHEMISTRY IN EVERYDAY LIFE
ARIHANT PUBLICATION|Exercise CHEMICAL IN INDUSTRIES (QUESTIONS FOR ASSESSMENT (MULTIPLE CHOICE TYPE QUESTIONS))|2 VideosCHEMISTRY IN EVERYDAY LIFE
ARIHANT PUBLICATION|Exercise CHEMICAL IN INDUSTRIES (QUESTIONS FOR PRACTICE (SHORT ANSWER TYPE QUESTIONS))|11 VideosCHEMICAL KINETICS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Long Answer Type Questions)|8 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE Long Answer Type Questions 7 MARKS|6 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-CHEMISTRY IN EVERYDAY LIFE -CHEMICAL IN INDUSTRIES (QUESTIONS FOR PRACTICE (SHORT ANSWER TYPE II QUESTIONS))
- Account for the: Some builders are added to soap.
Text Solution
|
- Account for the: Using soap is safer than detergents from the envi...
Text Solution
|
- Account for the: Ethanol is added to soaps.
Text Solution
|
- Explain the Cationic detergents with suitable examples.
Text Solution
|
- Explain the Anionic detergents with suitable examples.
Text Solution
|
- Explain the Non-ionic detergents with suitable examples.
Text Solution
|
- Label the hydrophilic and hydrophobic parts in the following compounds...
Text Solution
|
- Label the hydrophilic and hydrophobic parts in the following compounds...
Text Solution
|
- Label the hydrophilic and hydrophobic parts in the following compounds...
Text Solution
|
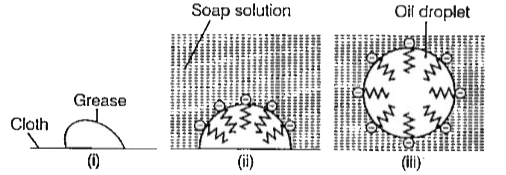
- Explain the cleansing action of soaps.
Text Solution
|