Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-EXAMINATION PAPER 2018-GROUP C
- State and exlain Kohlarausch.s law of independent migration of ions . ...
Text Solution
|
- Write the assumptions of crystal field theory. Discuss the patterm of ...
Text Solution
|
- Define an expression for the rate constant of a 1st order reaction. De...
Text Solution
|
- How can you distinguish between primary, secondary and tertiary alcoho...
Text Solution
|
- How can you distinguish between primary ,secondary and tertiary alcoho...
Text Solution
|
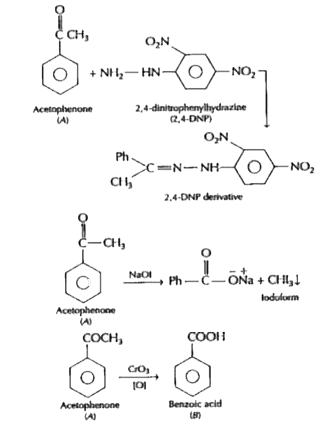
- An organic compound (A) with molecular formula C8H8O forms an orange p...
Text Solution
|
- What is Willámson synthesis?
Text Solution
|
 .
.