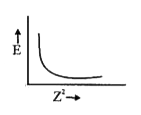
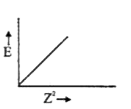
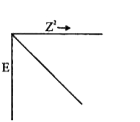
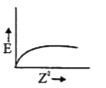
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - II|87 VideosSTRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - II (ASSERTION - REASON)|5 VideosSTATES OF MATTER
BRILLIANT PUBLICATION|Exercise LEVEL III (Linked Comprehension Type )|9 VideosTHERMODYNAMICS AND CHEMICAL ENERGETICS
BRILLIANT PUBLICATION|Exercise (LEVEL-II (HOMEWORK)|39 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-STRUCTURE OF ATOMS-LEVEL - III (Linked Comprehension Type)
- The energy of an electron moving in n^(th) Bohr's orbit of an element ...
Text Solution
|
- The hydrogen-like species Ļi^(2+) is in a spherically symmetric state ...
Text Solution
|
- The hydrogen-like species Ļi^(2+) is in a spherically symmetric state ...
Text Solution
|
- The hydrogen-like species Ļi^(2+) is in a spherically symmetric state ...
Text Solution
|
- Werner Heisenberg considered the limits of how precisely we can measur...
Text Solution
|
- Werner Heisenberg considered the limits of how precisely we can measur...
Text Solution
|
- For one-electron species, the wave number of radiation emitted during ...
Text Solution
|
- For one-electron species, the wave number of radiation emitted during ...
Text Solution
|
- The wavelength (in cm) of second line in the Lyman series of hydrogen ...
Text Solution
|