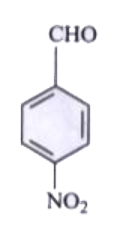
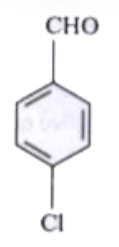
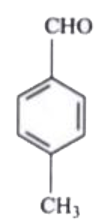
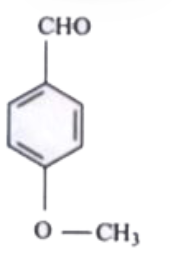
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY: BASIC PRINCIPLES - PART II (REACTION MECHANISM)
BRILLIANT PUBLICATION|Exercise LEVEL -II |45 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES - PART I (NOMENCLATURE)
BRILLIANT PUBLICATION|Exercise LEVEL-II |41 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES - PART III (PURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUND)
BRILLIANT PUBLICATION|Exercise LEVEL - II (Assertion Reason type Question)|8 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-ORGANIC CHEMISTRY: BASIC PRINCIPLES - PART II (REACTION MECHANISM)-LEVEL -II
- The carbon-chlorine bond length is shortest in
Text Solution
|
- Which of the following carbocation is least stable?
Text Solution
|
- In which of the following pairs the second member is more stable than ...
Text Solution
|
- Arrange the following In the increasing order of stability
Text Solution
|
- Which of the following free radical is most stable?
Text Solution
|
- The stability order of the following carbocations is
Text Solution
|
- The decreasing order of reactivity towards electrophilic substitution ...
Text Solution
|
- Which is wrong statement?
Text Solution
|
- Arrange the following halides in the increasing order of SN^2 reactivi...
Text Solution
|
- Chlorination of toluene in presence of sunlight is an example of
Text Solution
|
- Addition of HCN to carbonyl compounds is
Text Solution
|
- The most reactive of the following towards nucleophilic addition is
Text Solution
|
- B^(Theta) + R - X to B - R + X^(Theta). The reaction is
Text Solution
|
- The main product of the reaction CH3 - underset(underset(CI)|)CH - CH2...
Text Solution
|
- When 3,3 -dimethyl '-2' -butanol is heated with 'H2 SO4', the major pr...
Text Solution
|
- Assertion : 1-butene on reaction with HBr presence of peroxide produce...
Text Solution
|
- Assertion : Tricyclopropyl methyl carbonium ion is more stable than tr...
Text Solution
|
- Assertion : m-chlorobenzoic acid is a stronger acid than p-chlorobenzo...
Text Solution
|
- Assertion : Allyl phenyl ether on heating rearranges to O-allyl phenol...
Text Solution
|
- Assertion : Cyanides R - C -= N: are very much weaker bases than aliph...
Text Solution
|