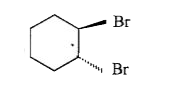
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-HYDROCARBON-LEVEL II
- , P will have configuration:
Text Solution
|
- Identify the incorrect statement : C7 H16 has nine structural isomer...
Text Solution
|
- Which among the following is the best method for the preparation of pu...
Text Solution
|
- A mixture of tert - Butyl chloride and Ethyl chloride is treated with ...
Text Solution
|
- Identify the wrong statement
Text Solution
|
- The acid which undergoes fastest decarboxylation with soda lime among ...
Text Solution
|
- Which of the following alkenes does not exhibit geometrical isomerism?
Text Solution
|
- Identify the false statement
Text Solution
|
- In which of the following reactions the major product given is not cor...
Text Solution
|
- Identify the wrong statement regarding alkenes : Heat of hydrogenat...
Text Solution
|
- Which one of the following alkenes will react fastest with H(2) under ...
Text Solution
|
- To which of the following compounds HBr or Br2 adds most readily? : ...
Text Solution
|
- Identify the wrong statement regarding addition of Br2//"CCI"4 to alke...
Text Solution
|
- In which of the following reaction the major product given is correct?
Text Solution
|
- An alkene on reductive ozonolysis gives CH2 (CHO)2. The alkene is
Text Solution
|
- Ozonolysis of an organic compound gives formaldehyde as one of the pro...
Text Solution
|
- Oxidation of an alkene X gives a diol and further oxidation gives a di...
Text Solution
|
- Identify the wrong statements
Text Solution
|
- A and B respectively are
Text Solution
|
- tert-butyl chloride can be converted to isobutene by
Text Solution
|
- For the following reaction the moles of (x) of alc.KOH consumed
Text Solution
|
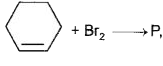 , P will have configuration:
, P will have configuration: