Text Solution
Verified by Experts
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-I|55 VideosSOME BASIC CONCEPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION REASON TYPE QUESTIONS)|7 VideosSOME BASIC CONCAPTS OF CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|6 VideosSTATES OF MATTER
BRILLIANT PUBLICATION|Exercise LEVEL III (Linked Comprehension Type )|9 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-SOME BASIC CONCEPTS OF CHEMISTRY-QUESTIONS
- Calculate the percentage of Cu in CuO
Text Solution
|
- Calculate the mass % of oxygen in Fe(3)O(4)
Text Solution
|
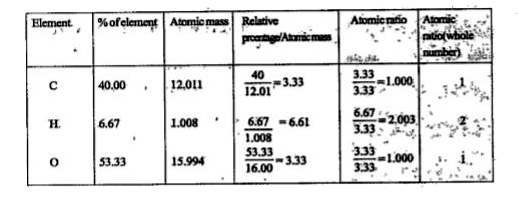
- An organic compound contains 40% carbon, 6.67% hydrogen and 53.33% oxy...
Text Solution
|
- Calculate the mass of CaO formed from 25g of CaCO(3) on strong heating...
Text Solution
|
- Calculate the volume of CO2 evolved at STP when 10g of CaCO3 is treate...
Text Solution
|
- What is the volume of H2 produced by the decomposition of 5L of NH3 at...
Text Solution
|
- Find out the limiting reagent when 5g of H2 reacts with 24g of O2 to f...
Text Solution
|
- Construct an ionic equation for the neutralisation of HNO3 and KOH.
Text Solution
|
- Calculate the mass percent of a solution containing 2.5g of KCl in 50 ...
Text Solution
|
- Calculate the molarity of a solution containing 2.8g of KOH per litre ...
Text Solution
|
- 250mL of 0.2M H(2)SO(4) is mixed with 100mL of 0.5M H(2)SO(4). What is...
Text Solution
|
- What is the final molarity of a solution of 9.8g H(2)SO(4) in 250mL wa...
Text Solution
|
- How much water should be added to 3M HCl to get 1L 0.25M HCl?
Text Solution
|
- Calculate the molarity of 10% by mass KCl in water solution (molecular...
Text Solution
|
- The density of 2.5 M acetic acid in water is 1.02g ml^(-1). Calculate...
Text Solution
|
- What is the normality of a solution of 0.285 mol of NaOH dissolved in ...
Text Solution
|
- 50 mL acid is titrated against 0.25N base. It took 22.3 mL of base to ...
Text Solution
|
- What is the mole fraction of the solute in a 1m aqueous solution?
Text Solution
|
- What is the mole fraction of C Cl4 in solution if 2.3 moles of C Cl4 i...
Text Solution
|
- What is the concentration in ppm, of a solution containing 50mg of Hg ...
Text Solution
|