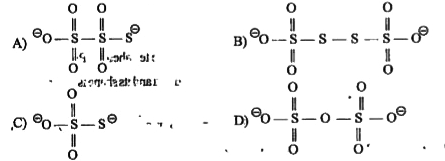
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-II|100 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|20 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-III ( Linked Comprehension Type )|12 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Linked Comprehension Type Questions)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL BONDING-LEVEL-I
- In PO4^(3-), P-O bond order is : 1.25, 2, -0.75, -3
Text Solution
|
- In which of the following species the bonds are non-directional? : N...
Text Solution
|
- There is no S - S bond in : S2O6^(2-), S4O6^(2-), S2O3^(2-), S2O7...
Text Solution
|
- Among the following the electron-deficient compound is
Text Solution
|
- The nodal plane in the pi-bond of ethene is located in: The molecula...
Text Solution
|
- Which of the following is a positive overlap which leads to bonding?
Text Solution
|
- The correct order of dipole moment is
Text Solution
|
- Which of the following hydrocarbons has the lowest dipole moment?
Text Solution
|
- The compound in which carbon uses sp^3 hybrid orbitals for bond format...
Text Solution
|
- SF2, SF4 and SF6 have the hybridisation at sulphur atom respectively a...
Text Solution
|
- Which of the following compounds has the least tendency to form H-bond...
Text Solution
|
- The bond angles of NH3, NH4^(o+) and overset(ѳ)NH2 are in the order:
Text Solution
|
- Resonance structures can be written for
Text Solution
|
- The bond length of C-=O bond in CO is 1.20 Å and in CO2 it is 1.34 Å. ...
Text Solution
|
- Which of the following is not diamagnetic?
Text Solution
|
- Which of the following molecules would be expected to be square planar...
Text Solution
|
- All the following species have all their bond lengths identical except
Text Solution
|
- According to the molecular orbital model, the highest occupied molecul...
Text Solution
|
- Which kind of attractive forces are likely to be holding particles tog...
Text Solution
|
- In which of the following pairs , the two species are not isostructura...
Text Solution
|