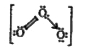A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-II|100 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|20 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-III ( Linked Comprehension Type )|12 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Linked Comprehension Type Questions)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL BONDING-LEVEL-I
- Which of the following compounds has the least tendency to form H-bond...
Text Solution
|
- The bond angles of NH3, NH4^(o+) and overset(ѳ)NH2 are in the order:
Text Solution
|
- Resonance structures can be written for
Text Solution
|
- The bond length of C-=O bond in CO is 1.20 Å and in CO2 it is 1.34 Å. ...
Text Solution
|
- Which of the following is not diamagnetic?
Text Solution
|
- Which of the following molecules would be expected to be square planar...
Text Solution
|
- All the following species have all their bond lengths identical except
Text Solution
|
- According to the molecular orbital model, the highest occupied molecul...
Text Solution
|
- Which kind of attractive forces are likely to be holding particles tog...
Text Solution
|
- In which of the following pairs , the two species are not isostructura...
Text Solution
|
- Which of the following species exhibits the diamagnetic behaviour?
Text Solution
|
- MgSO4 is soluble while BaSO4 is insoluble in H2O. This is because
Text Solution
|
- Which pair is not correct order of lattice energy ?
Text Solution
|
- Select incorrect statement.
Text Solution
|
- Which of the following statements regarding Hg^(2+) (ionic radius 116 ...
Text Solution
|
- The increasing order of covalency in silver halides is
Text Solution
|
- Which of the following pairs of species are not isostructural?
Text Solution
|
- Select incorrect statement.
Text Solution
|
- Which of the following statements regarding I(3)^(-) is not correct?
Text Solution
|
- Which of the following statements is correct?
Text Solution
|