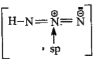A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|20 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise QUESTIONS|6 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-I|120 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Linked Comprehension Type Questions)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL BONDING-LEVEL-II
- CO2 is a gas at room temperature while SiO2 is a high melting solid. T...
Text Solution
|
- The nitrite ion NO2^(-) may be respresented by two major resonance for...
Text Solution
|
- Among the following species, identify the isostructural pairs : NF3, N...
Text Solution
|
- Arrange the following compounds in the order of increasing dipole mome...
Text Solution
|
- The cyanide ions CN^(ѳ) and N2 are isoelectronic, but in contrast to C...
Text Solution
|
- The correct order of hybridisation of the central atom in the followin...
Text Solution
|
- The common features among the species CN^(ѳ), CO and NO^(o+) are : ...
Text Solution
|
- The percentage of 'p character in the orbitals forming P-P bonds in 'P...
Text Solution
|
- For which of the following molecules, mu ne 0?
Text Solution
|
- The ionic radii of isoelectronic species 'N^(3-), O^(2-)' and 'F^-' in...
Text Solution
|
- Element X is strongly electropositive and element Y is strongly electr...
Text Solution
|
- Polarising action of Cd^(2+) on anions is stronger than that of Ca^(2+...
Text Solution
|
- PCl5 has a shape of trigonal bipyramid whereas IF5 has the shape of a ...
Text Solution
|
- Amolecule of the type AX5 has square pyramidal geometry. Hence, number...
Text Solution
|
- Hybridization of the nitrogen atom and electron geometry around nitrog...
Text Solution
|
- H2O molecule is dipolar, whereas BeF2 is non polar. It is because
Text Solution
|
- Which of the following compounds contains the maximum number of lone p...
Text Solution
|
- Which of the following molecules contains the maximum S-O bond length?...
Text Solution
|
- What are the formal charges of the atoms in the nitrite ion in the ord...
Text Solution
|
- Molecule AB has a bond length of 1.67 Å and a dipole moment of 0.38 D....
Text Solution
|