A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
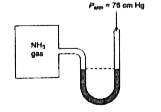
- A manometer attached to a flask contains ammonia gas have no differenc...
Text Solution
|
- Figure shows a manometer, one arm is connected with a bulb containing ...
Text Solution
|
- The partial pressure of hydrogen in a flask containing 2 g of H(2) and...
Text Solution
|
- A manometer attached to a flask contains with ammonia gas have no diff...
Text Solution
|
- A monometer contains a liquid of density 5.44g//cm^(3) is attached to ...
Text Solution
|
- A manometer attached to a flask contains with ammonia gas have no diff...
Text Solution
|
- For the reaction N(2(g))+3H(2(g))rarr 2NH(3(g)) the rate of the reacti...
Text Solution
|
- A manometer attached to a flask contains NH(3) gas have no difference ...
Text Solution
|
- A sample of HI(g) is placed in flask at a pressure of 0.2 atm. At equi...
Text Solution
|