A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following reaction: 2 NO (2) (g) to 2 NO (g) + O (2) (g)...
Text Solution
|
- Consider the following reaction: 2NO(2)(g) rarr 2NO(g)+ O(2)(g) In the...
Text Solution
|
- Consider the following reaction : xMnO(4)^(-)+yC(2)O(4)^(2-)+zH^(+) to...
Text Solution
|
- The following steps are involved in finding the value of (a^(x+y))^(x-...
Text Solution
|
- Prove that |{:(x^(2),,x^(2)-(y-z)^(2),,yz),(y^(2),,y^(2)-(z-x)^(2),,zx...
Text Solution
|
- निम्नलिखित अभिक्रिया पर विचार कीजिये: xMnO(4)^(-)+yC(2)O(4)^(2-)+zH...
Text Solution
|
- Consider the following reaction: xMnO(4)^(-)+yC(2)O(4)^(2-)+zH^(+) t...
Text Solution
|
- SO(2)(g)+Cl(2)(g) to X overset(P(4)) to Y+Z then X, Y and Z can be :
Text Solution
|
- A factor o |((a-x)^(2),(b-x)^(2),(c-x)^(2)),((a-y)^(2),(b-y)^(2),(c-y)...
Text Solution
|
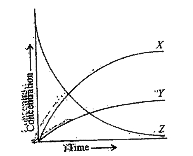 : `X = NO , Y =O _(2) , Z = NO _(2)` , `X = O _(2) Y = NO , Z = NO _(2)` , `X = NO _(2), Y = NO, Z = O _(2)` , `X = O _(2), Y = NO _(2), Z = NO`
: `X = NO , Y =O _(2) , Z = NO _(2)` , `X = O _(2) Y = NO , Z = NO _(2)` , `X = NO _(2), Y = NO, Z = O _(2)` , `X = O _(2), Y = NO _(2), Z = NO`