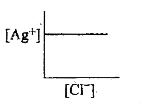
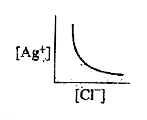
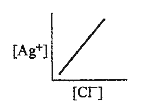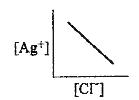A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a saturated solution of AgCl, addition of NaCl is made drop by drop...
Text Solution
|
- In a saturated solution of AgCl, NaCl is added gradually the concentra...
Text Solution
|
- In a saturated solution of AgCl, NaCl is added gradually. The concentr...
Text Solution
|
- The addition of NaCl to AgCl decreases the solubility of AgCl because
Text Solution
|
- A litre of solution is saturated with AgCl. To this solution if 1.0 xx...
Text Solution
|
- Addition of excess AgNO(3) to NaCl solution gives positively charged A...
Text Solution
|
- A litre of solution is saturated with AgCl To this solution if 1.0 × 1...
Text Solution
|
- The addition of NaCl to AgCI decreases the solubility of AgCl, because
Text Solution
|
- In NaCl is doped with 1 xx 10^(-3) mol percent of SrCl(2) , what is th...
Text Solution
|