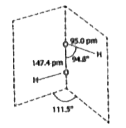
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The O-O-H bond angle in H2O2 in the gas phase is
Text Solution
|
- O-O-H bond angle in H2O2 is approximately.
Text Solution
|
- H2O2 के अणु में H - O - H कोण होता है-
Text Solution
|
- The bond angle between C-O and O-H bonds in alcohols is close to
Text Solution
|
- The O-O-H bond angle in H2 O2 is ?
Text Solution
|
- H2O2 णु में O - H तलों के बीच कोण होता है
Text Solution
|
- In H2O2 molecule, the H-O-O angle is
Text Solution
|
- In H2O2 molecule, the O-O bond length is
Text Solution
|
- Bond angle (H-O-H) in H(2)O is
Text Solution
|