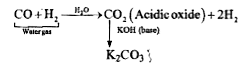A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In context with the industrial preparation of hydrogen from water gas ...
Text Solution
|
- In context with the industrial preparation of hydrogen from water gas ...
Text Solution
|
- In context with the industrial preparation of hydrogen from water gas ...
Text Solution
|
- In context with the industrial preparation of hydrogen from water gas ...
Text Solution
|
- In context with the industrial preparation of hydrogen from water gas ...
Text Solution
|
- The reaction of , water gas (CO+H2)+H2 at 673 K, 300 atmosphere in pre...
Text Solution
|
- Answer the following : Mixture of CO and H2 is used for preparation ...
Text Solution
|
- In context with the industrial preparation of hydrogen from water gas ...
Text Solution
|
- In context with the industrial preparation of hydrogen from water gas...
Text Solution
|