A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART II (ISOMERISM AND REACTION MECHANISM)
BRILLIANT PUBLICATION|Exercise LEVEL-II|40 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART II (ISOMERISM AND REACTION MECHANISM)
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|20 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART I (NOMENCLATURE)
BRILLIANT PUBLICATION|Exercise LEVEL-III|45 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART III (PURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS)
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|15 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART II (ISOMERISM AND REACTION MECHANISM) -LEVEL-II (ASSERTION-REASON TYPE)
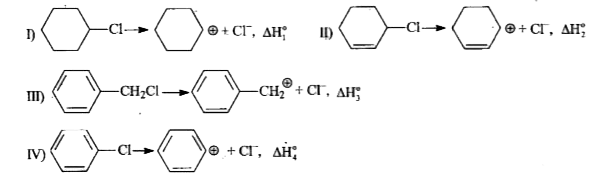
- The decreasing order of enthalpies of reaction for producing carbocati...
Text Solution
|
- Assertion : Simple carbanions are usually pyramidal but allyl carbanio...
Text Solution
|
- Assertion : All the carbon atoms of but-2-ene lie in one plane. Reas...
Text Solution
|
- Assertion : A free radical is paramagnetic species. Reason : A free ...
Text Solution
|
- Assertion : Tertiary carbocations are generally formed more easily tha...
Text Solution
|
- Assertion : tert-Butyl carbanion is more stable than methyl carbanion....
Text Solution
|
- Assertion : The order of stability of carbocations are R(3)C^(+)gtR(2)...
Text Solution
|
- Assertion : 1^(@) allylic halides are more reactive than 1^(@) RX in S...
Text Solution
|
- Assertion : Crown ether acts as phase transfer catalysis and increases...
Text Solution
|
- Assertion : RS^(Theta) is a stronger nucleophile and a better leaving ...
Text Solution
|
- Assertion : E(1)cB reaction is favoured by stabilisation of carbanion ...
Text Solution
|
- Assertion : Inductive effect is responsible for the dipole moment in t...
Text Solution
|
- Assertion : A carbanion is pyramidal in shape like NH(3) and amines. ...
Text Solution
|
- Assertion : Hybridisation influences the bond length and bond enthalpy...
Text Solution
|
- Assertion : Rotation about C = C is restricted. Reason : Electron ch...
Text Solution
|
- Assertion : Heterolytic fission involves breaking of bond in such a wa...
Text Solution
|
- Assertion : The order of reactivity of carbocations is 3^(@)gt2^(@)1^(...
Text Solution
|
- Assertion : When inductive and electromeric effects operate in opposit...
Text Solution
|
- Assertion : 1^(@) allylic halides are more reactive than 1^(@) RX in S...
Text Solution
|
- Assertion : Rate of ethanolysis of 1^(@) halide by S(N)1 mechanism is...
Text Solution
|
- Assertion : Allyl and benzyl carbonium ions are more stable than propy...
Text Solution
|