A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART II (ISOMERISM AND REACTION MECHANISM)
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|20 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART II (ISOMERISM AND REACTION MECHANISM)
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|20 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART I (NOMENCLATURE)
BRILLIANT PUBLICATION|Exercise LEVEL-III|45 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART III (PURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS)
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|15 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART II (ISOMERISM AND REACTION MECHANISM) -LEVEL-II
- Which of the following reactions will not give Hofmann alkene?
Text Solution
|
- Consider the following carbanions? Correct order of stability is
Text Solution
|
- Which of the following explain why propene undergo electrophilic addit...
Text Solution
|
- Examine the following statements regarding S(N)2 reaction 1. The rat...
Text Solution
|
- The substitution reaction among the following is
Text Solution
|
- Which among the following statements are true with respect to electron...
Text Solution
|
- The arrangement of (CH(3))(3)C-,(CH(3))(2)CH-,CH(3)CH(2)- when attache...
Text Solution
|
- The stability of Me(2)C=CH(2) is more than that of MeCH(2)CH=CH(2) due...
Text Solution
|
- Among the following structure the one which is not a resonating struct...
Text Solution
|
- The stability of carbanions in the order: I. RC-=C II. III. R(2)C=C...
Text Solution
|
- The effective electrophile in aromatic sulphonation is:
Text Solution
|
- Among the given compounds, the most susceptible to nucleophilic attack...
Text Solution
|
- Which one of the nitrogen atoms in underset("I")(H(2)N)-underset("II")...
Text Solution
|
- Which one of the following is an intermediate in the reaction of benze...
Text Solution
|
- The hydrolysis of 2-bromo-3-methylbutane by S(N)1 mechanism gives main...
Text Solution
|
- In an S(N)2 substitution reaction of the type R-Br+Cl^(-)overset("DMF"...
Text Solution
|
- The product of which of the following reactions has the highest ease t...
Text Solution
|
- Which will undergo fastest S(N)2 substitution reaction when treated wi...
Text Solution
|
- Under identical conditions, S(N)1 reaction will occur most efficient w...
Text Solution
|
- Consider the reaction, RCHO+NH(2)NH(2)rarrRCH=N-NH(2). What type of re...
Text Solution
|
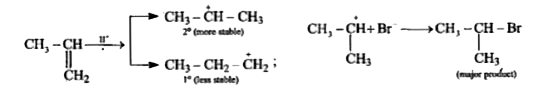 `CH_(3)-undersetunderset(CH_(3))(|)overset(+)(CH)+Br^(-)rarrunderset(("major product"))(CH_(3)-underset(CH_(3))underset(|)(CH)-Br)`
`CH_(3)-undersetunderset(CH_(3))(|)overset(+)(CH)+Br^(-)rarrunderset(("major product"))(CH_(3)-underset(CH_(3))underset(|)(CH)-Br)`