A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL - II) (Assertion - Reason)|4 VideosCHEMICAL KINETICS
BRILLIANT PUBLICATION|Exercise QUESTION|19 VideosCHEMICAL KINETICS
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL - l) (HOMEWORK) (ASSERTION- REASON) |6 VideosCARBOXYLIC ACIDS
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion -Reason)|4 VideosCO-ORDINATION COMPOUNDS AND ORGANOMETALLICS
BRILLIANT PUBLICATION|Exercise Level-II (Assertion- Reason)|4 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL KINETICS -QUESTION (LEVEL - II)
- In a reaction 2A + B to 3C, the concentration of A decreases from 0.5...
Text Solution
|
- For the reaction: 2A+B→A 2 B, the rate =k[A[B]2 with k=2.0×10^−6mol^−...
Text Solution
|
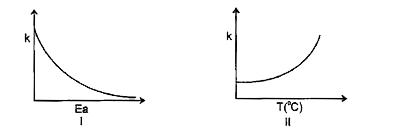
- Consider the given plots for a reaction obeying Arrhenius equation (0°...
Text Solution
|
- For an elementary chemical reaction, A(2) overset (k(1)) underset(k(-...
Text Solution
|
- If I is, the intensity of absorbed light and 'C' is the concentration ...
Text Solution
|
- The reaction of ozone with oxygen atoms in the presence of chlorine at...
Text Solution
|
- The rate law for the reaction below is given by the expression k[A][B]...
Text Solution
|
- For the reaction A + 2B to C + D , the following data were obtained: ...
Text Solution
|
- The following mechanism has been proposed for the reaction of (NO) wit...
Text Solution
|
- Consider the reaction, 2A + B to Products When concentrations of B ...
Text Solution
|
- Higher order (gt 3) are rare due to :
Text Solution
|
- The slope of plot of [A] versus t for a zero-order reaction A to B is...
Text Solution
|
- For the reaction A to products, is zero order w.r.t. A. The half-life...
Text Solution
|
- For the first-order decomposition reaction of N(2)O(5) written as: 2...
Text Solution
|
- The gaseous reaction A(g) to 2B(g) + C(g) is found to be first-order r...
Text Solution
|
- The radioactive decay follows
Text Solution
|
- The hydrolysis of an ester was carried out separately with 0.05 M HCI ...
Text Solution
|
- The reaction 2NO(g) + H(2)(g) to N(2)O(g) + H(2)O(g) follows the rate...
Text Solution
|
- For a second-order reaction of the type rate = k[A]^2, the plot of 1//...
Text Solution
|
- The half-life of a second-order reaction is
Text Solution
|