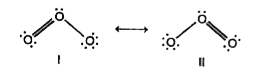A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS (XII)
BRILLIANT PUBLICATION|Exercise LEVEL- II (GROUP 15 ELEMENTS (PNICOGENS)) |13 VideosTHE P-BLOCK ELEMENTS (XII)
BRILLIANT PUBLICATION|Exercise LEVEL- II (GROUP 16 OXYGEN FAMILY)|10 VideosTHE D-AND F-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-III)|50 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-THE P-BLOCK ELEMENTS (XII) -LEVEL- II (GROUP 18 NOBLE GASES) (ASSERTION - REASON)
- O(3) molecule is a resonance hybrid of the two structure I and II ...
Text Solution
|
- Assertion: N2 is less reactive than P Reason: Nitrogen gas more nega...
Text Solution
|
- Assertion: SF6 can't be hydrolysed but SF4 can be. Reason: Six fluor...
Text Solution
|
- Assertion: HI cannot be prepared by the reaction of Kl with concentrat...
Text Solution
|
- Assertion: Xenon is the only group 18 element which form compounds. ...
Text Solution
|
- Assertion: O3 is a better oxidant than H2O2 Reason: The E^(0) of O(...
Text Solution
|