A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
BRILLIANT PUBLICATION|Exercise Level-II|37 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL - II (ASSERTION - REASON) |5 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-HALOALKANES AND HALOARENES-Level-II
- The rate of reaction with AgNO(3) will be :
Text Solution
|
- Which is incorrect in the physical properties of halogen compounds ?
Text Solution
|
- Which one of the following is not the correct order of boiling points ...
Text Solution
|
- Which among the following has highest density ?
Text Solution
|
- Which of the following reaction is known as Hunsdiecker reaction ?
Text Solution
|
- Alkyl halides can be prepared by the following reaction except
Text Solution
|
- 2CH(3)-CH(2)-Cl+Hg(2)F(2)to 2CH(3)-CH(2)-F+Hg(2)Cl(2) . This reaction ...
Text Solution
|
- The function of halogen carrier (catalyst) in halogenation of benzene ...
Text Solution
|
- Neopentyl alcohol on treatment with HBr gives (1) neopentyl bromide ...
Text Solution
|
- Which of the following combinations is correctly matched
Text Solution
|
- A dextrorotatory optically active alkyl halide undergo hydrolysis by S...
Text Solution
|
- Which of the following compounds is most soluble in water?
Text Solution
|
- In which of the following solvent S(N)1 reaction is maximum
Text Solution
|
- overset ("NaI/Acetone")(to) P. The product is:
Text Solution
|
- Which is the correct reaction coordinate diagram for the following sol...
Text Solution
|
- When concentration of alkyl halide is tripled and the concentration of...
Text Solution
|
- CH(3)-overset(CH(3))overset(|)(CH)-CH(2)-CH(3)overset(Cl(2),hv)(to)C(5...
Text Solution
|
- (-)2- methylbutan- 1 - ol on heating with conc. HCl gives (+)1- chloro...
Text Solution
|
- 1,3 dichloro propane reacts with zinc or Nal gives (major product)
Text Solution
|
- Which of the following is most reactive towards E(2) reaction
Text Solution
|
- Dehydrobromination of the following is in the order (I)
Text Solution
|
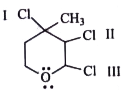 The rate of reaction with `AgNO_(3)` will be :
The rate of reaction with `AgNO_(3)` will be :