A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL II|50 VideosP-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL II (ASSERTION AND REASON)|20 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type) |11 VideosPOLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL -III (Comprehension Type) |12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-P-BLOCK ELEMENTS-LEVEL-III
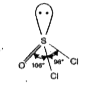
- In SOCl2 , the Cl-S-Cl and Cl-S-O bond angles are
Text Solution
|
- Which of the following is the correct statement
Text Solution
|
- A dark brown solid (X) reacts with NH3 to form a mild explosive which ...
Text Solution
|
- Which of the following formulae represents peroxodisulphuric acid?
Text Solution
|
- The structure of SO(3)^(2-) is
Text Solution
|
- The products obtained when iodine reacts with hot and concentrated sol...
Text Solution
|
- A gas “X” is passed through water to form a saturated solution. The aq...
Text Solution
|
- Which of the following statements regarding interhalogens is not corre...
Text Solution
|
- In a sealed nickel vessel, Xe and F2 were taken in 1:20 volume ratio a...
Text Solution
|
- The acidic strength of oxoacids of chlorine is in the order 'HClO4,HCl...
Text Solution
|
- Select correct statement(s).
Text Solution
|
- Which of the following orders are incorrect?
Text Solution
|
- Which of the following statements are not correct?
Text Solution
|
- Which statements are correct?
Text Solution
|
- The nitrogen containing compound produced in the reaction of HNO3 with...
Text Solution
|
- A solution of colourless salt Honboiling with excess NaOH produces a n...
Text Solution
|
- The correct statement(s) about O3 is (are)
Text Solution
|
- Which of the following hydrogen halides react(s) with AgNO3 (aq) to gi...
Text Solution
|
- The correct statement(s) regarding (i) HClO, (ii) HClO2, (iii) HClO3 a...
Text Solution
|
- The nitrogen oxide(s) that contains(s) N-N bond(s) is (are)
Text Solution
|
- Which of the following statements are incorrect?
Text Solution
|