A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Arrange the following compounds in the increasing order of their densi...
Text Solution
|
- Which of the following arrangements does not represent the correct ord...
Text Solution
|
- निम्नांकित में कौन सत्य है ? (i) sin90^(@) lt sin 60^(@) lt sin45^(@...
Text Solution
|
- The correct solubility order is/are I. CaCO3 gt SrCO3 gt BaCO3 II...
Text Solution
|
- A random variable X has the following probability distribution : ...
Text Solution
|
- A random variable X has the following probability distribution: ...
Text Solution
|
- A random variable X has the following probability distribution: ...
Text Solution
|
- Which of the following arrangements represent the correct order of the...
Text Solution
|
- A random variable X has the following probability distribution: ...
Text Solution
|
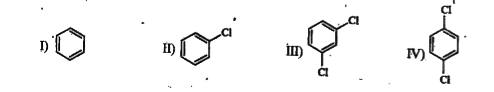 `I lt II lt III lt IV`,
`I lt III lt IV lt II`,
`IV lt III lt II lt I`,
`II lt IV lt III lt I`
`I lt II lt III lt IV`,
`I lt III lt IV lt II`,
`IV lt III lt II lt I`,
`II lt IV lt III lt I`