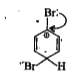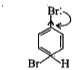A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
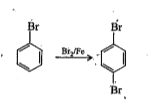
- Assertion: Bromobenzene upon reaction with Br2//Fe gives 1,4-dibromobe...
Text Solution
|
- Assertion: Bromobenzene upon reaction with Br(2)//Fe gives 1,4-dibromo...
Text Solution
|
- Assertion: Phenol is more reactive than benzene towards electrophilic ...
Text Solution
|
- Assertion: Acetanilide is more reactive than aniline towards electroph...
Text Solution
|
- Assertion: Bromobenzene, upon reaction with Br(2)//Fe gives 1,4-dibrom...
Text Solution
|
- Statement-1:Bromobenzene upon reaction with Br2//Fe gives 1,4-dibromob...
Text Solution
|
- Statement-1: Bromobenzene upon reaction with Br(2)//Fe gives 1,4-dibro...
Text Solution
|
- Statement-1. Bromobenzene upon reaction with Br2 /Fe gives 1. 4-dibrom...
Text Solution
|
- Assertion. Propene is more reactive than ethene towards electrophilic ...
Text Solution
|