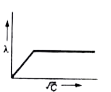
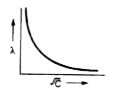
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
BRILLIANT PUBLICATION|Exercise Questions|17 VideosELECTROCHEMISTRY
BRILLIANT PUBLICATION|Exercise Level -I|50 VideosELECTROCHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-I|35 VideosD & F BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise Level -II|38 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL - II (ASSERTION - REASON) |5 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-ELECTROCHEMISTRY-LEVEL-II
- 1 L of 1 MCuSO(4) solution is electrolysed. After passing 2 F of elec...
Text Solution
|
- The resistance of 0.1 N solution of salt is found to be 2.5 xx 10^(3) ...
Text Solution
|
- The variation of equivalent conductance of weak electrolyte with conce...
Text Solution
|
- A solution of sodium sulphate in water is electrolyzed using inert ele...
Text Solution
|
- Which of the following condition will increase the voltage of the cell...
Text Solution
|
- What is wrongly stated about electrochemical series?
Text Solution
|
- Which of the following statements is true for the Daniel cell?
Text Solution
|
- Which of the following does not evolve oxygen at anode when electrolys...
Text Solution
|
- A current of 4 A is passed through a solution of silver nitrate to coa...
Text Solution
|
- In the electrolysis of copper (II) chloride solution, the mass of cath...
Text Solution
|
- Which of the following expressions is correct?
Text Solution
|
- A conductivity cell whose cell constant is 3.0 cm^(-1) is filled with...
Text Solution
|
- The conductivity of a saturated solution of a sparingly soluble salt, ...
Text Solution
|
- Which of the following statements regarding the movement of ions in a ...
Text Solution
|
- Efficiency of a fuel cell is 80% and the standard heat of reaction is ...
Text Solution
|
- A current of 965 ampere is passed for 1 sec through 1 litre solution o...
Text Solution
|
- Given that K(sp) of CuS= 10^(-35) and E(Cu2+ //Cu)^(@)=0.34 V The s...
Text Solution
|
- Cost of electricity for the production of xL H(2) at NTP at cathode i...
Text Solution
|
- The equilibrium constant of the following redox reaction at 298 K is 1...
Text Solution
|
- At 25^(@)C the molar conductances at infinite dillution for the stron...
Text Solution
|