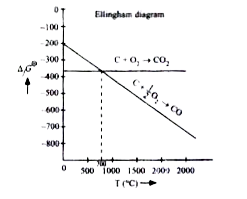
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is incorrect on the basis of the given Ellingha...
Text Solution
|
- Which of the following is incorrect on the basic of the above Ellingha...
Text Solution
|
- On the basis of ellingham diagram which of the following is not correc...
Text Solution
|
- Consider the following Ellingham diagram for carbon Which of the ...
Text Solution
|
- Which of the following statemetnt is incorrect regarding Ellingham dia...
Text Solution
|
- On the basis of Ellingham diagram which of the following is/are correc...
Text Solution
|
- Which of the following statements is INCORRECT on the basis of Ellingh...
Text Solution
|
- On the basis of below given diagram select the incorrect statement -
Text Solution
|
- In the Ellingham diagram, for the formation of carbon monoxide
Text Solution
|