A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE D- AND F-BLOCK ELEMENT
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|40 VideosTHE D- AND F-BLOCK ELEMENT
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE)|40 VideosSURFACE CHEMISTRY
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL - II) (ASSERTION REASON TYPE QUESTIONS) |6 VideosTHE D-AND F-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-III)|50 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-THE D- AND F-BLOCK ELEMENT-LEVEL-II
- The atomic numbers of vanadium(V), chromium (Cr), manganese (Mn) and i...
Text Solution
|
- The radius of La^3+. is 1.06 Å, which of the following given values wi...
Text Solution
|
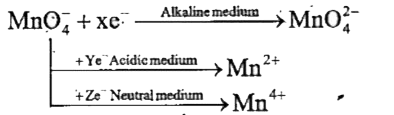
- X, Y and Z are respectively :
Text Solution
|
- Zinc gives H2" gas with " H2SO4, and conc. HCl" but not with conc. HN...
Text Solution
|
- The highest magnetic moment is shown by the transition metal ion with ...
Text Solution
|
- Number of moles of K2Cr2O7, reduced by one mole of Sn^(2+) ions is:
Text Solution
|
- A blue solution of copper sulphate becomes darker when treated with ex...
Text Solution
|
- An inorganic compound (A) gave the following reactions: The compound ...
Text Solution
|
- In context with the transition elements, which of the following statem...
Text Solution
|
- Iron exhibits +2 and +3 oxidation states. Which of the following about...
Text Solution
|
- Which of the following arrangements does not represent the correct ord...
Text Solution
|
- Chloro compound of vanadium has only spin magnetic moment of 1.73 BM. ...
Text Solution
|
- Which of the following is not formed when H2S , reacts with acidic K2...
Text Solution
|
- Which of the following reaction(s) can be used for the complete conver...
Text Solution
|
- Consider the following complex ions P, Q and R: P=[FeF6]^(3-), Q=[V(...
Text Solution
|
- Which one of the following does not correctly represent the correct or...
Text Solution
|
- Consider the following statements in respect of lanthanides: The basi...
Text Solution
|
- The electronic configuration of Cu(II)is 3d^9 whereas that of Cu(I) is...
Text Solution
|
- Interstitial compounds are formed when small atoms are trapped inside ...
Text Solution
|
- Although Zirconium belongs to 4d transition series and Hafnium to 5d t...
Text Solution
|