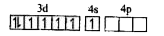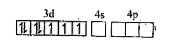A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL I |55 VideosCOORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL II|50 VideosCOORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-II|50 VideosCO-ORDINATION COMPOUNDS AND ORGANOMETALLICS
BRILLIANT PUBLICATION|Exercise Level-II (Assertion- Reason)|4 VideosD & F BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise Level -II|38 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-COORDINATION COMPOUNDS -LEVEL-II (ASSERTION- REASON TYPE )
- Assertion : [Co(NO(2))3(NH3)3] does not show optical isomerism. Reas...
Text Solution
|
- Assertion : [Ti(H2O)6]^(3+) is coloured while [Sc(H2O)6]^(3+) is colou...
Text Solution
|
- Assertion : [Ni(CN)4]^(2-) is square planar and diamagnetic. Reason ...
Text Solution
|
- Assertion : In crystalline solids, the value of resistance is differen...
Text Solution
|
- Assertion : [Fe(CN)6]^(3-) is weakly paramagnetic while [Fe(CN)6]^(4-)...
Text Solution
|
- Assertion : Aqueous solution of the compound CoCl3. 4 NH3 when treated...
Text Solution
|
- Assertion : The complex K3 [Cr(C2O4)3] when present in aqueous soluti...
Text Solution
|
- Assertion : Tetrahedral complexes having two different types of uniden...
Text Solution
|
- Assertion : Inner orbital complexes are low spin complexes. Reason :...
Text Solution
|
- Assertion : According to crystal field theory, during complex formatio...
Text Solution
|
- Assertion : [Fe(H2O)6]^(3+) is strongly paramagnetic whereas [Fe(CN)6]...
Text Solution
|
- Assertion : EDTA is a hexadentate ligand. Reason : Denticity of a li...
Text Solution
|
- Assertion . : The [Ni(en)3]Cl3 has lower stability than [Ni(NH3)6]Cl. ...
Text Solution
|
- Assertion : Potassium ferrocyanide is diamagnetic whereas potassium fe...
Text Solution
|
- Assertion : Oxidation state of Fe in Fe(CO)3 is zero. Reason : EAN ...
Text Solution
|
- Assertion : When NO reacts with FeSO4, a brown coloured complex is for...
Text Solution
|
- Asserțion : [Fe(H2O)5 NO]SO4 is paramagnetic. Reason : The Fe in [Fe...
Text Solution
|
- Assertion : The geometrical isomers of the complex [M(NH3)4 Cl2] are o...
Text Solution
|
- Assertion : Complexes of MX6 and MX5L type (X and L are unidentate) do...
Text Solution
|
- Assertion : Co-ordination number of complex in [HgI3]^(-) , [Fe(CO)5] ...
Text Solution
|