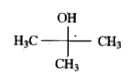
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOL, PHENOL & ETHER
BRILLIANT PUBLICATION|Exercise LEVEL II |100 VideosALCOHOL, PHENOL & ETHER
BRILLIANT PUBLICATION|Exercise LEVEL II (ASSERTION - REASON TYPE) |40 VideosALCOHOL PHENOL AND ETHERS
BRILLIANT PUBLICATION|Exercise LEVEL-II |40 VideosALCOHOLS, PHENOLS AND ETHERS
BRILLIANT PUBLICATION|Exercise LEVEL III (Linked Comprehension Type) |12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-ALCOHOL, PHENOL & ETHER -LEVEL II (ASSERTION - REASON TYPE)
- Among the following alcohols, which one is most acidic?
Text Solution
|
- Assertion : Carbon oxygen bond length of phenol is slightly less than ...
Text Solution
|
- Assertion : In alcohols, the boiling point decreases with decrease in...
Text Solution
|
- Assertion : Alcohols and water are weaker acids than phenols. Reas...
Text Solution
|
- Assertion : Bromination of phenol takes place even in the absence of...
Text Solution
|
- Assertion : The cleavage of C-O bond in ethers takes place under dra...
Text Solution
|
- Assertion: Dipolemoment ofphenol is smaller than that of methanol. R...
Text Solution
|
- Assertion: Neopentyl alcohol on treatment with HCl gives neopentyl chl...
Text Solution
|
- Assertion: Phenoxide ion on treatment with an active alkyl h...
Text Solution
|
- Assertion:Phenol forms 2,4,6-tribromophenol on treatment with Br2 in c...
Text Solution
|
- Assertion: Alcohols have higher boiling points than ethers of comparab...
Text Solution
|
- Assertion: Commercially carboxylic acids are reduced to alcohols by co...
Text Solution
|
- Assertion: Ethers are not prepared by the dehydration of secondary and...
Text Solution
|
- Assertion: CO2 is non polar, while ROR is polar in nature. Reason: ...
Text Solution
|
- Assertion: t-Butyl methyl ether is not prepared by the reaction of t-b...
Text Solution
|
- Assertion: Anisole undergoes electrophilic substitution at o-and p-pos...
Text Solution
|
- Assertion: Acid catalysed dehydration of tert-butanol proceeds faster ...
Text Solution
|
- Assertion: Reaction of alcohols with SOCl2 is catalysed by the presenc...
Text Solution
|
- Assertion: Solubility of n-alcohl in water decreases with increase in ...
Text Solution
|
- Assertion: The bond angle in alcohols is slightly less than the tetr...
Text Solution
|
- Assertion: Addition reaction of water to but-1-ene in acidic medium yi...
Text Solution
|