A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
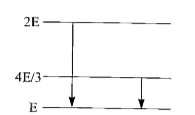
- The given diagram indicates the energy levels of a certain atom. When ...
Text Solution
|
- The energy levels of a cartain atom are shown in figure. If a photon o...
Text Solution
|
- The follwing diagram indicates the energy levels of a certain atom whe...
Text Solution
|
- The following diagram indicates the energy levels of a certain atom wh...
Text Solution
|
- सलग्न चित्र में किसी का ऊर्जा -स्तर प्रदर्शित है । जब परमाणु 2E से E ...
Text Solution
|
- The energy level of an atom for 1 ^(st),2 ^(nd) and third levels are E...
Text Solution
|
- दिया गया चित्र किसी परमाणु के ऊर्जा स्तरों को दर्शाता है। जब इलेक्ट्रॉ...
Text Solution
|
- चित्रानुसार जब कोई इलेक्ट्रॉन ऊर्जा स्तर 2E से ऊर्जा स्तर E में संक्रम...
Text Solution
|
- किसी परमाणु में ऊर्जा स्तर A से C में संक्रमण में 1000Å तथा ऊर्जा स्त...
Text Solution
|