A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD-THERMODYNAMICS-EXERCISE
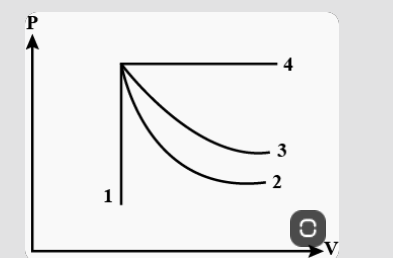
- An ideal gas undergoes four different processes from the same initial ...
Text Solution
|
- An ideal gas with pressure P, volume V and temperature T is expanded i...
Text Solution
|
- A litre of hydrogen at 27^@C and 10^5 Nm^-2 pressure expand isothermal...
Text Solution
|
- A volume of gas at atmospheric pressure is compressed adiabatically to...
Text Solution
|
- The efficiency of an ideal engine is 1/8. By lowering the temperature ...
Text Solution
|
- What thermodynamical variable is defined by Zeroth law
Text Solution
|
- Is it possible to convert internal energy into work?
Text Solution
|
- An ideal gas is compressed at a contant temperature. Will its internal...
Text Solution
|
- Does the internal energy of an ideal gas change in an isothermal proce...
Text Solution
|
- How is the efficiency of a Carnot engine affected by the nature of the...
Text Solution
|
- If the door of a refrigerator is kept open, the room in which the refr...
Text Solution
|
- Why first law of thermodynamics does not forbid flow of heat from lowe...
Text Solution
|
- What do you mean by "internal energy"?
Text Solution
|
- Fill in the Blanks: Air escaping from a cycle-tube becomes .
Text Solution
|
- Can two isothermal curves intersect each other? Why?
Text Solution
|
- What do you mean by reversible and irreversible process? Give example.
Text Solution
|
- Write two characterstics of a reversible process.
Text Solution
|
- State first law of thermodynamics.
Text Solution
|
- Show that PV^(gamma) = constant for adiabatic process.
Text Solution
|