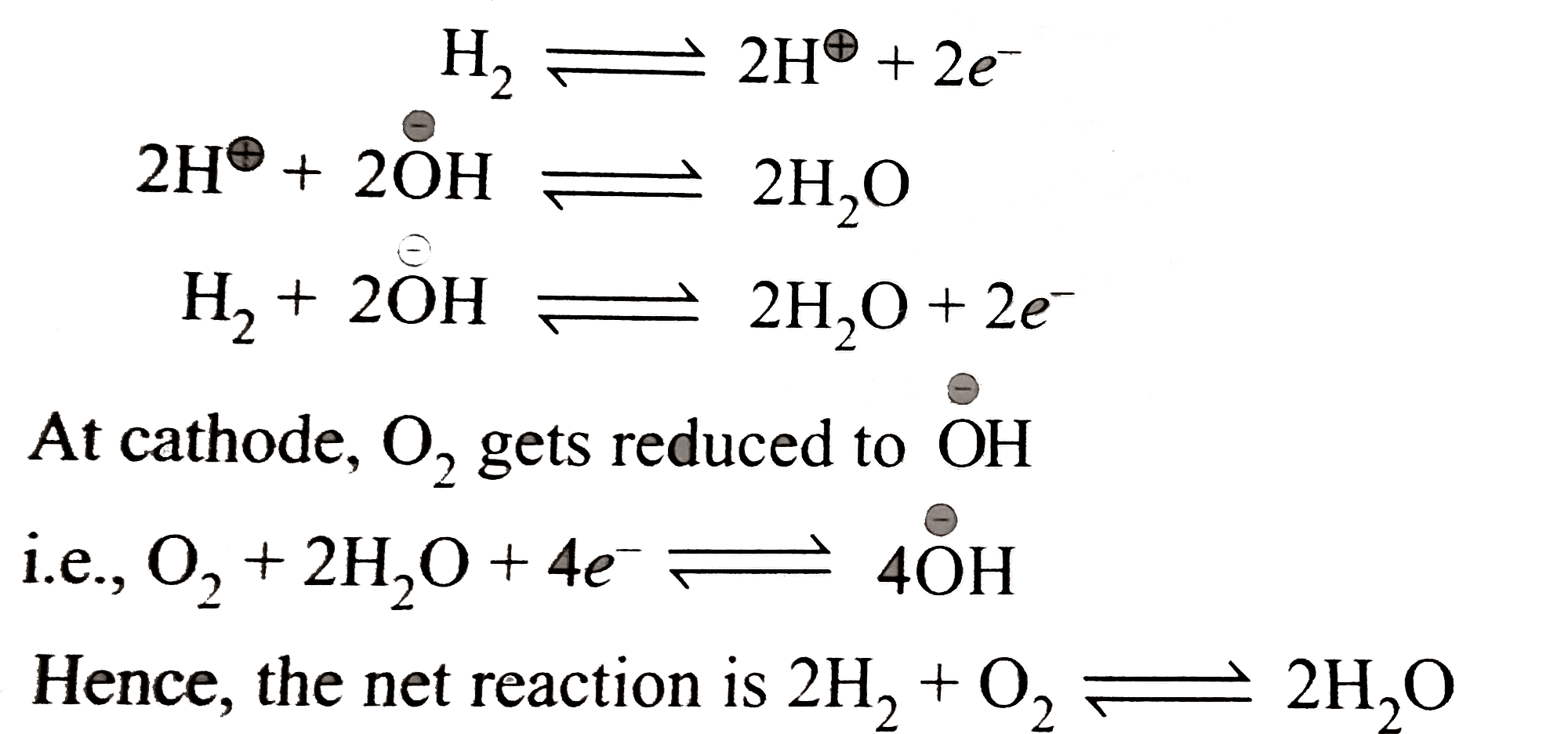A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- In a hydrogen-oxygen fuel cell, combustion of hydrogen occurs to :
Text Solution
|
- ईधन सेल क्या हैं। हाइड्रोजन –ऑक्सीजन ईधन सेल का वर्णन संक्षेप में कीज...
Text Solution
|
- Assertion: Galvanic cells containing hydrogen, methane, methanol etc. ...
Text Solution
|
- हाइड्रोजन - ऑक्सीजन ईधन सैल में हाइड्रोजन का दहन होता है -
Text Solution
|
- ईंधन सेल में ईंधन ऊर्जा को विद्युत ऊर्जा में बदला जाता है।
Text Solution
|
- Purpose of hydrogen -oxygen fuel cell is to
Text Solution
|