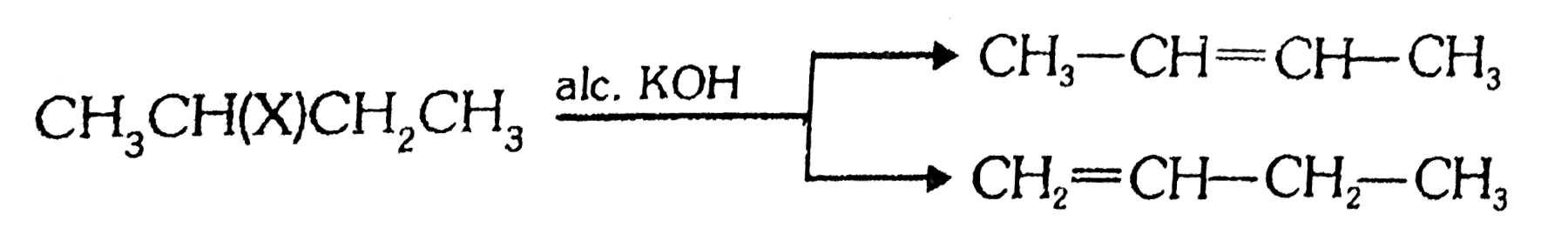
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the reaction
Text Solution
|
- Select the reactions involved in the reaction above reaction :
Text Solution
|
- किस अभिक्रिया में ताप वृद्धि से अग्र अभिक्रिया अधिक होगी?
Text Solution
|
- Displacement reactions, Combination reactions, Decomposition reactions...
Text Solution
|
- Displacement reactions, Combination reactions, Decomposition reactions...
Text Solution
|
- निम्न अभिक्रिया लिखिए - (i) कार्बिलऐमीन अभिक्रिया , (ii)आयडोफॉर्म अभ...
Text Solution
|
- Write the following reactions : (a) Haloform reaction (b) Gattermann r...
Text Solution
|
- Introduction to Oxidation Reaction | Reduction Reaction | Rearrangemen...
Text Solution
|
- Introduction to Substitution Reaction | Addition Reaction | Eliminatio...
Text Solution
|