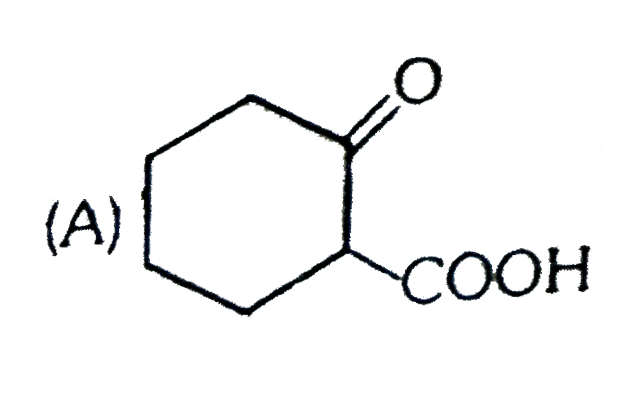
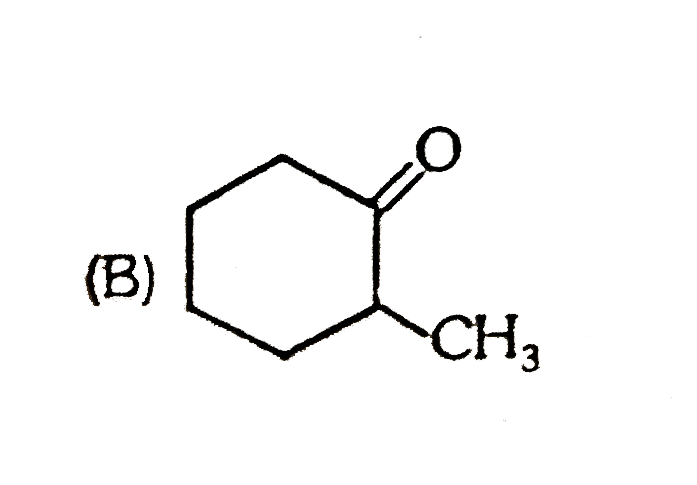
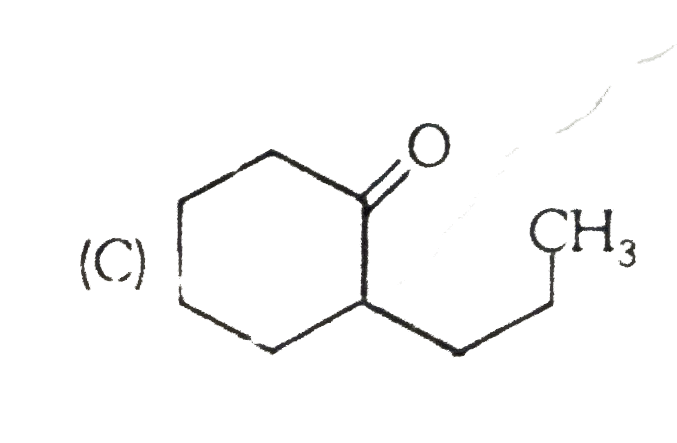
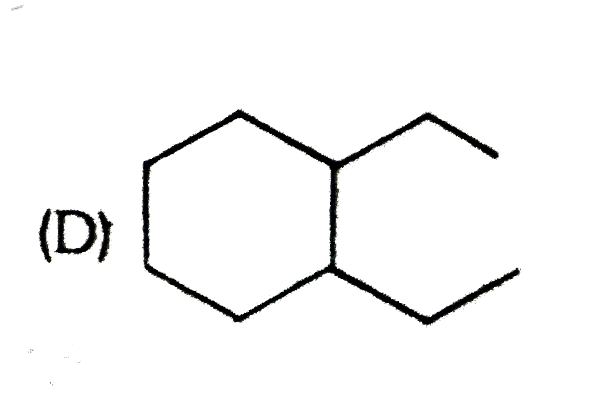
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An organic compound A has molecular formula C(10)H(17)Br and it is non...
Text Solution
|
- An organic compound A has molecular formula C(10)H(17)Br and it is non...
Text Solution
|
- An organic compound A has molecular formula C(10)H(17)Br and it is non...
Text Solution
|
- An organic compound A has molecular formula C(10)H(17)Br and it is non...
Text Solution
|
- An organic compound A has molecular formula C(10)H(17)Br and it is non...
Text Solution
|
- An alkyl bromide A has molecular formula C(8)H(17)Br and four differen...
Text Solution
|
- An organic compound 'A' has molecular formula C(9)H(13)NO and it can b...
Text Solution
|
- An organic compound 'A' has molecular formula C(9)H(13)NO and it can b...
Text Solution
|
- An organic compound 'A' has molecular formula C(9)H(13)NO and it can b...
Text Solution
|