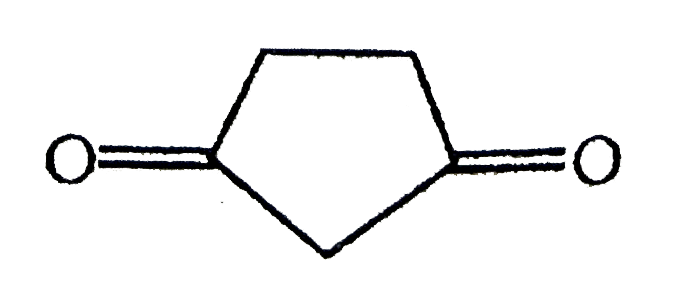
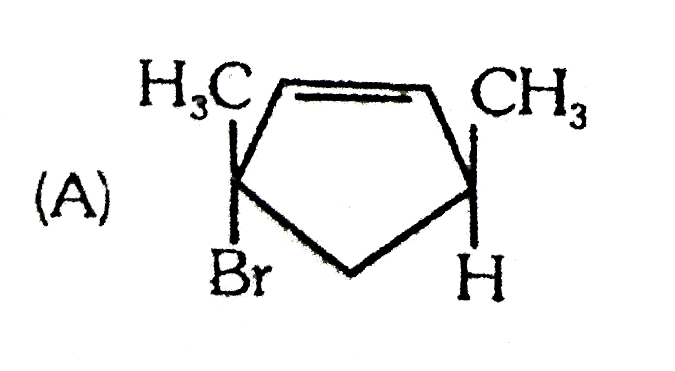
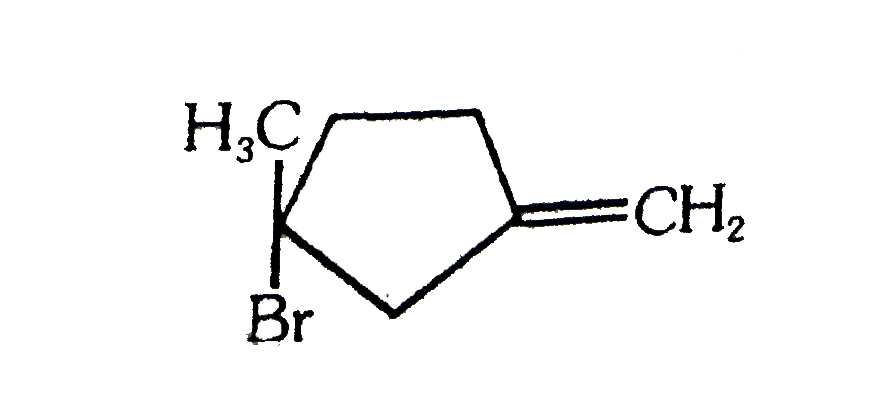
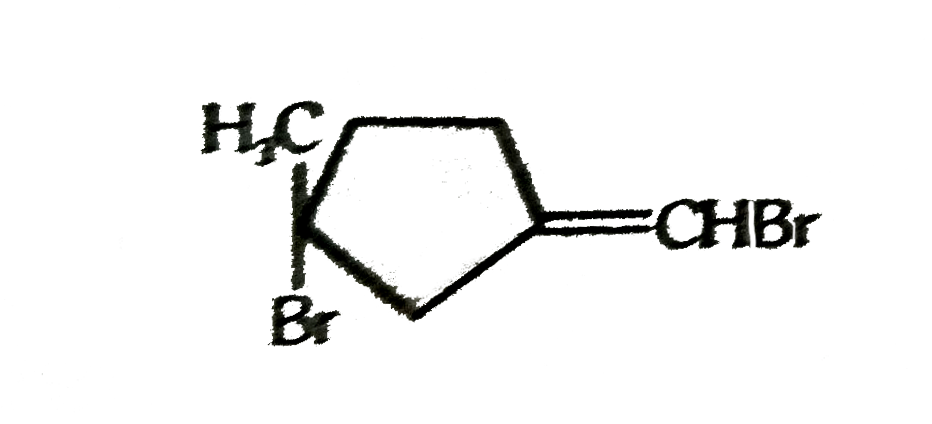
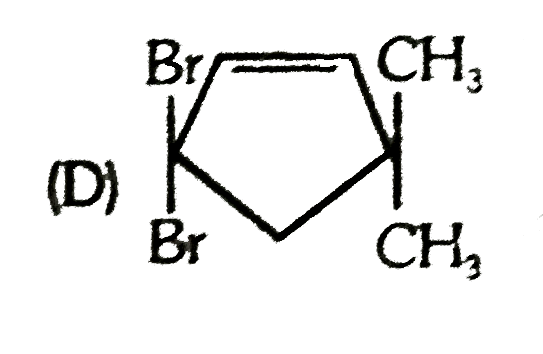A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An optically active compound A (assume dextrorotatory) has the molecul...
Text Solution
|
- An alkyl bromide A has molecular formula C(8)H(17)Br and four differen...
Text Solution
|
- A organic compound A has molecular formula C(11)H(14)O.A on treatment ...
Text Solution
|
- A compound [A] of molecular formula C(4)H(9)Br yields a compound [B] o...
Text Solution
|
- When potassium tert-butoxide and methyl bromide are heated, the produc...
Text Solution
|
- An aromatic compound 'A' having molecular formula C(7)H(6)O(2) on trea...
Text Solution
|
- An aromatic compound 'A' having molecular formula C(7)H(6)O(2) on trea...
Text Solution
|
- A compound with molecular formula C(7)H(16) shows optical isomerism, t...
Text Solution
|
- Compound A of molecular formula C(2)H(8) is treated with chlorine and ...
Text Solution
|