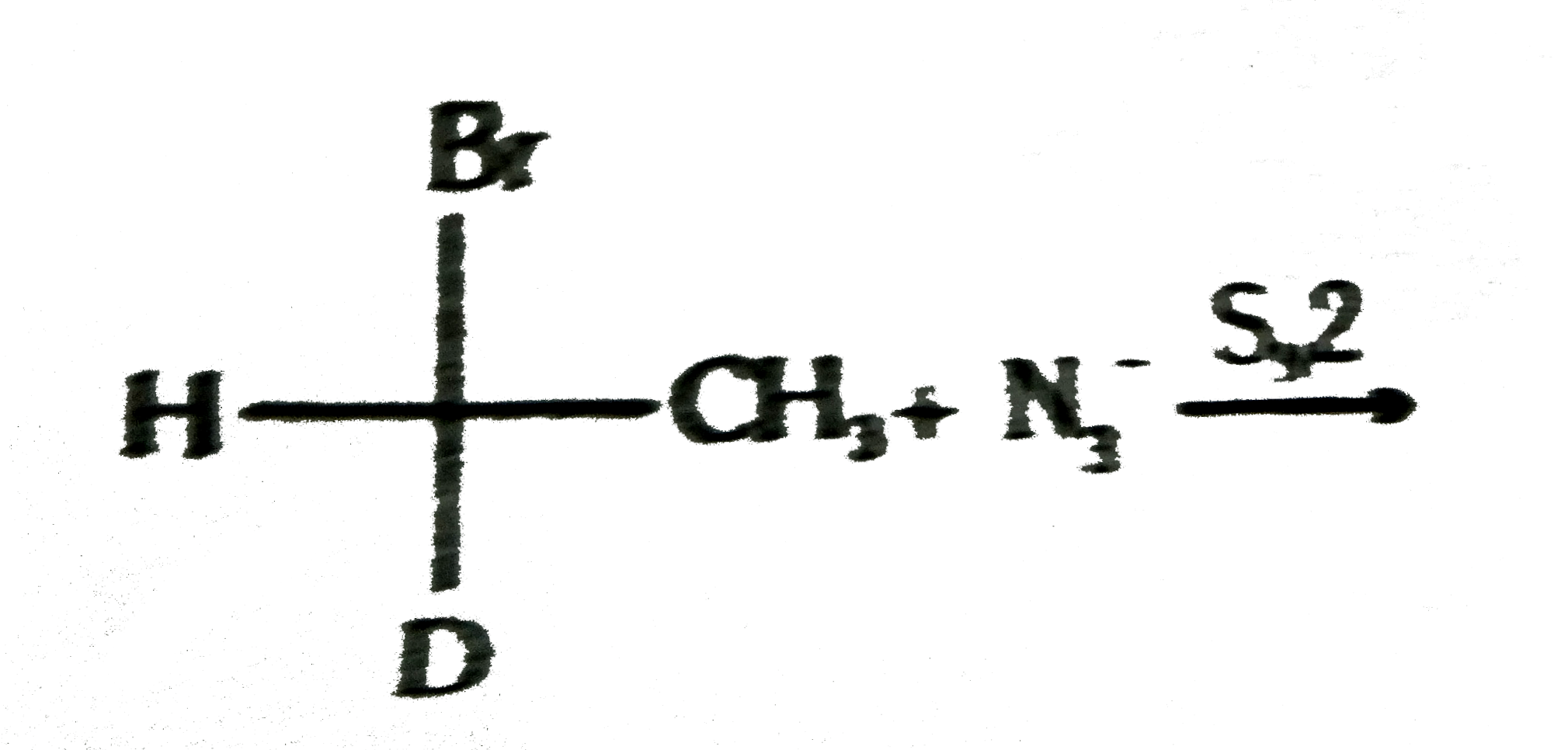Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain the following observations: (a) Azide ion (N(3)) react with ...
Text Solution
|
- Of the following statements which are true for S(N)1 reaction. (a) Ter...
Text Solution
|
- Assertion (A) : Neopentyl chloride undergoes S(N^(2)) reaction easily....
Text Solution
|
- Which will react faster in S(N^(2)) displacement reaction, 1-Bromopent...
Text Solution
|
- Among the choices of alkyl bromide , the least reactive bromide in a S...
Text Solution
|
- In the reaction : R-Br+CI^(-) to R -CI+Br^(-) The rates of react...
Text Solution
|
- Give reason for the following: Ethyl iodide undergoes S(N)2 reaction f...
Text Solution
|
- Arrange the following compounds in order of incraesing S(N)2 reacitivi...
Text Solution
|
- What happens when an alkyl halide reacts with AgNO(2) and the product ...
Text Solution
|